Faster Recovery.
Less Pain.
Through therapeutic vibration VibeTech is the leader in helping those in need of strength recover faster with less work.
FDA Listed Technology
INTRODUCING THE VIBETECH 3
VibeTech has taken what it learned from the treatment of thousands of patients and turned it into its smartest device yet, the VibeTech 3.
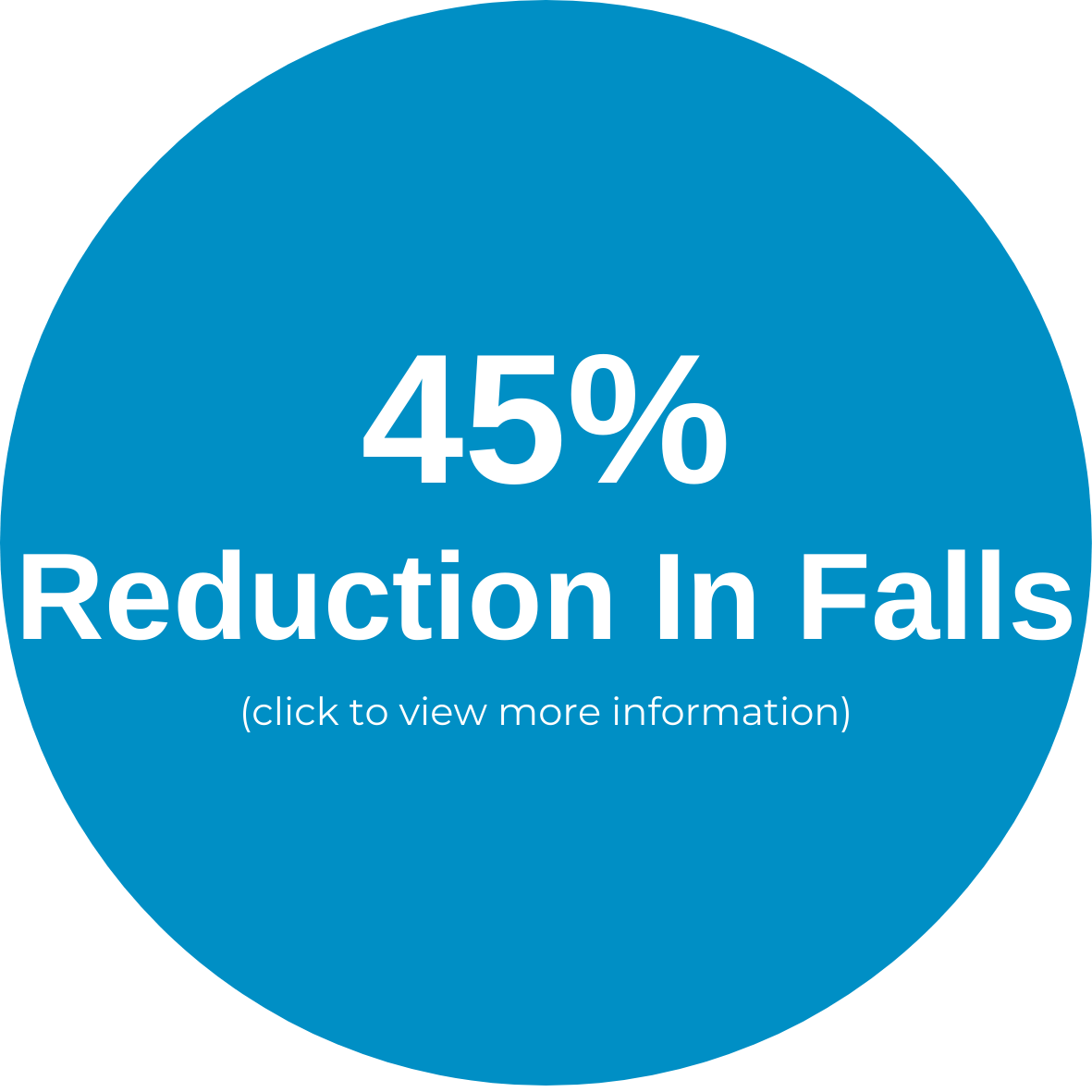
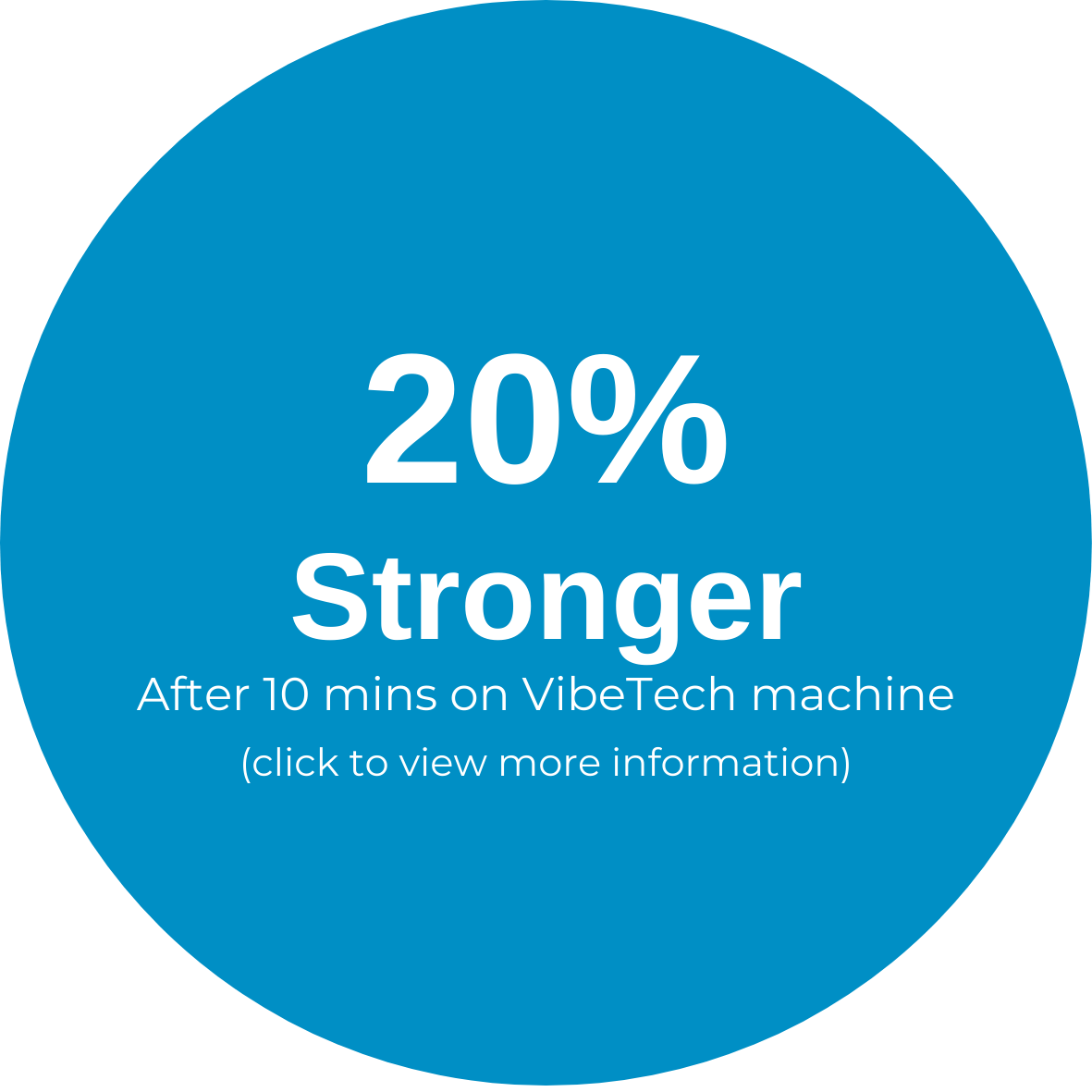
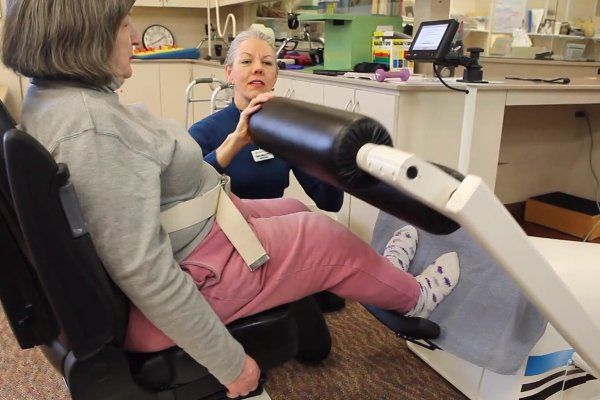
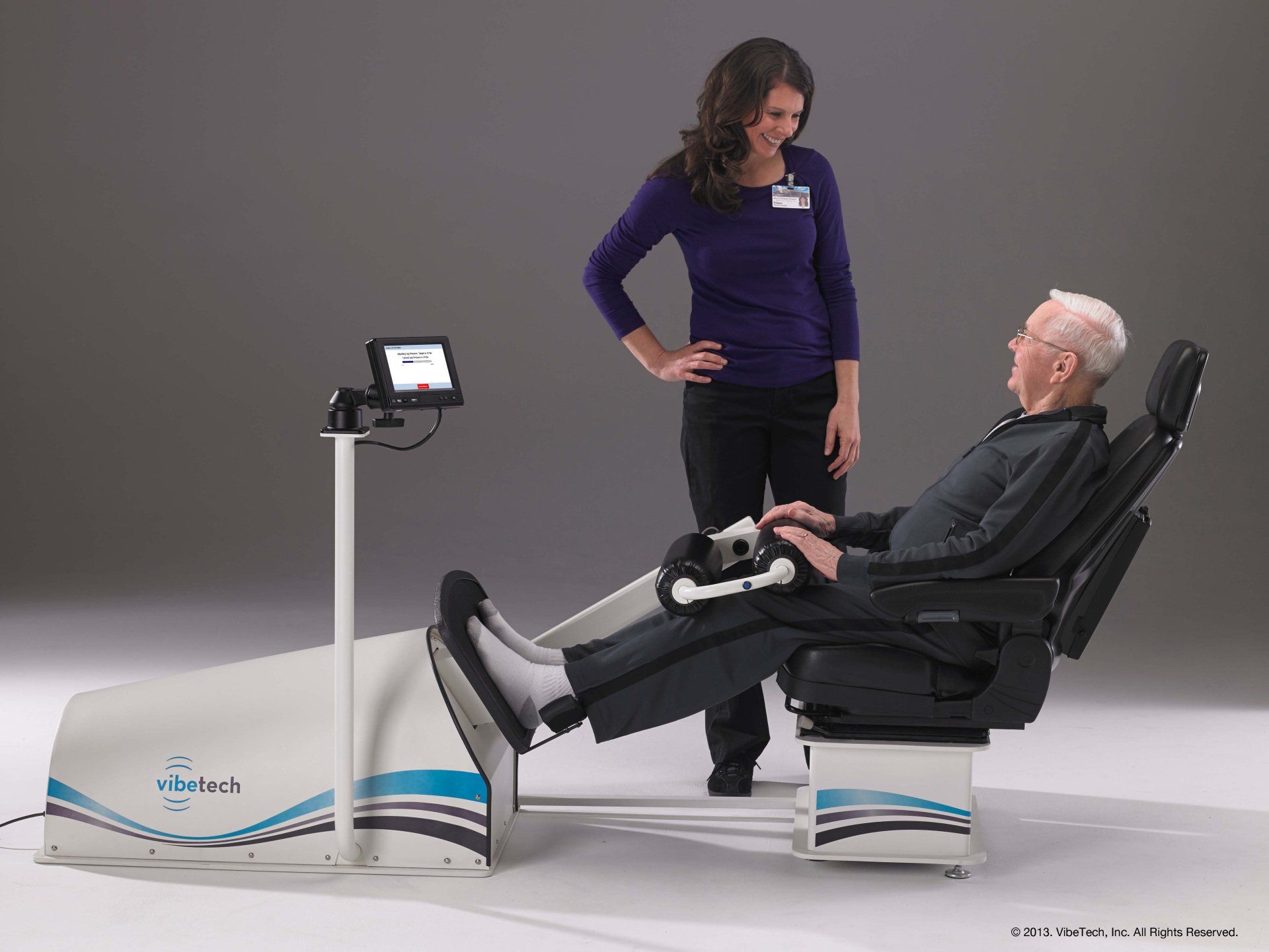
The VibeTech 3 is VibeTech’s answer to the call to reduce falls in the highest risk older adults. It activates the muscles throughout the legs using safe and effective, patient-specific (AutoDosing™) therapeutic vibration. Treatment is delivered through a footplate to a seated user. There is no transfer needed for wheelchair users. This portable device can be disinfected and brought to individual patient rooms or it can be placed in a rehab gym or other common space to boost strength and function while reducing pain in the lower extremities.
It is intended for both rehabilitative and restorative care purposes. Treatments with VibeTech’s technology are safe, effective, accessible, and reimbursable, and they have been shown to reduce falls, boost strength, elevate mood, and reduce pain in older adults with mobility impairments (as well as those living with Alzheimer's disease who are twice as likely to fall as other nursing home residents).
Unlike electrical stimulation, therapeutic vibration does not impart electricity into the body. Rather, it replicates minute mechanical signaling that occurs every time a step is taken as well as every time the muscles contract. When taking steps and performing physical activity is difficult or impossible due to physical or cognitive decline, the VibeTech 3 enables the user to rapidly progress, reversing the effects of dysmobility to reduce the risk of falls and fall-related injuries.
W H O I S V I B E T E C H
OVER 20,000 PATIENT TREATMENTS COMPLETE
The VibeTech family trusts our product can change your life, increase mobility, reduce falls, and get you back on your feet.
VibeTech brings safe, accessible physical therapy and occupational therapy to people with impaired physical mobility. The device can be used while the user is seated, with or without user effort.
VibeTech technology is patented and FDA registered. It is designed to address issues of individual patients, help therapists, nurses, and other staff with their work and make it easy for facilities, centers and hospitals to spread smiles along with financial and other benefits. Impaired physical mobility, pain and other neuromuscular conditions pose difficult problems in the lives of many and needs careful attention.
W H Y V I B E T E C H
OUR SOLUTIONS
100% passive therapy for individuals with extremely low
levels of weight bearing tolerance and range of motion.
V I B E T E C H A W A R D S
AWARDED MOST INNOVATIVE IN WISCONSIN 2017
Unleashing the potential of therapeutic vibration -delivering faster recovery with less pain.
VibeTech has been operating a research and demonstration site in Sheboygan, Wisconsin since the summer of 2018. In that time, we have had
nearly 50 visitors comprised of healthy individuals, people with mobility impairments, people recovering from injury/surgery, individuals looking to build strength before surgery, people with neuropathy,
people looking to reduce pain in their feet, legs, and low back, and more. We captured data from every user. Below are some highlights from this work.
W H O V I B E T E C H H E L P S
WE SERVICE PATIENTS WITH MOBILITY ISSUES
We know our product can change your life, increase mobility, reduce falls, and get you back on your feet.
With the introduction of the new VibeTech 3 portable therapeutic vibration device to nursing homes, activities centers, assisted living facilities, group homes, memory care facilities and hospitals in communities throughout the United States, VibeTech is poised to offer training and assistance in demonstrating how to collaborate to provide the most effective fall reduction program available to people living with cognitive impairments and others who are at a heightened risk of falls throughout the continuum of care.
VIBETECH NEWS AND UPDATES
VIBETECH IN THE NEWS
VIBETECH POSTS & UPDATES

CONTACT VIBETECH
Have A Representative Reach Out
Interested in learning about VibeTech or want to buy one? Get In touch with us using the form below.